

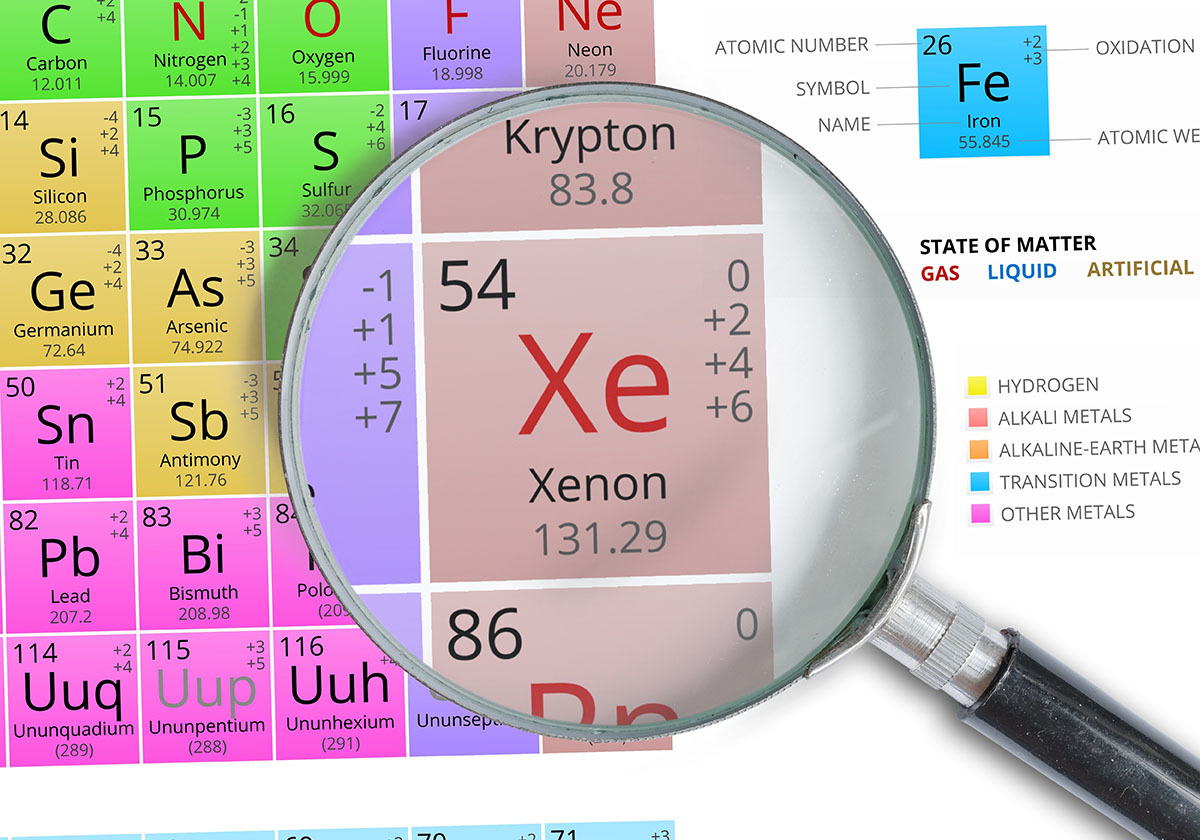
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a colourless, odourless, and tasteless noble gas. It is the second heaviest of the noble gases, and it is the rarest of the naturally occurring noble gases.
Xenon is found in trace amounts in the atmosphere, and it can also be extracted from natural gas. It is a very inert gas, and it does not react with other elements under normal conditions.
Xenon is used in a variety of applications, including:
Xenon is used in flashbulbs and lasers.
Noun:
xenon (a colourless, odourless, inert gaseous element).
The word "xenon" comes from the Greek word "xenos" (stranger). The first recorded use of the word "xenon" in English was in 1898.
What is xenon used for?
Question:
Explain the significance of xenon in various applications, including its use in lighting technology. Discuss the unique properties of xenon that make it suitable for certain purposes.
Answer:
Xenon, a noble gas, finds essential applications in various fields, particularly in lighting technology. Xenon gas discharge lamps, commonly used in vehicle headlights and high-intensity lighting, rely on the unique properties of xenon to produce intense illumination.
In xenon gas discharge lamps, an electric current passes through xenon gas, ionizing it and causing the gas to emit a brilliant, white light. Unlike traditional incandescent bulbs, xenon lamps do not rely on a heated filament, resulting in less energy loss as heat and longer bulb lifetimes. This makes them highly efficient for high-intensity applications.
One of xenon's exceptional characteristics is its ability to emit light across a broad spectrum, closely resembling natural sunlight. This property is particularly advantageous for enhancing visibility and reducing eye strain in applications such as automotive headlights.
Furthermore, xenon is chemically inert, making it safe and suitable for various environments. Its lack of reactivity allows for stable, long-lasting light emission in gas discharge lamps without deterioration over time.
Address
Developing Experts Limited
Exchange Street Buildings
35-37 Exchange Street
Norwich
NR2 1DP
UK
Phone
01603 273515
Email
hello@developingexperts.com
Copyright 2025 Developing Experts, All rights reserved.