

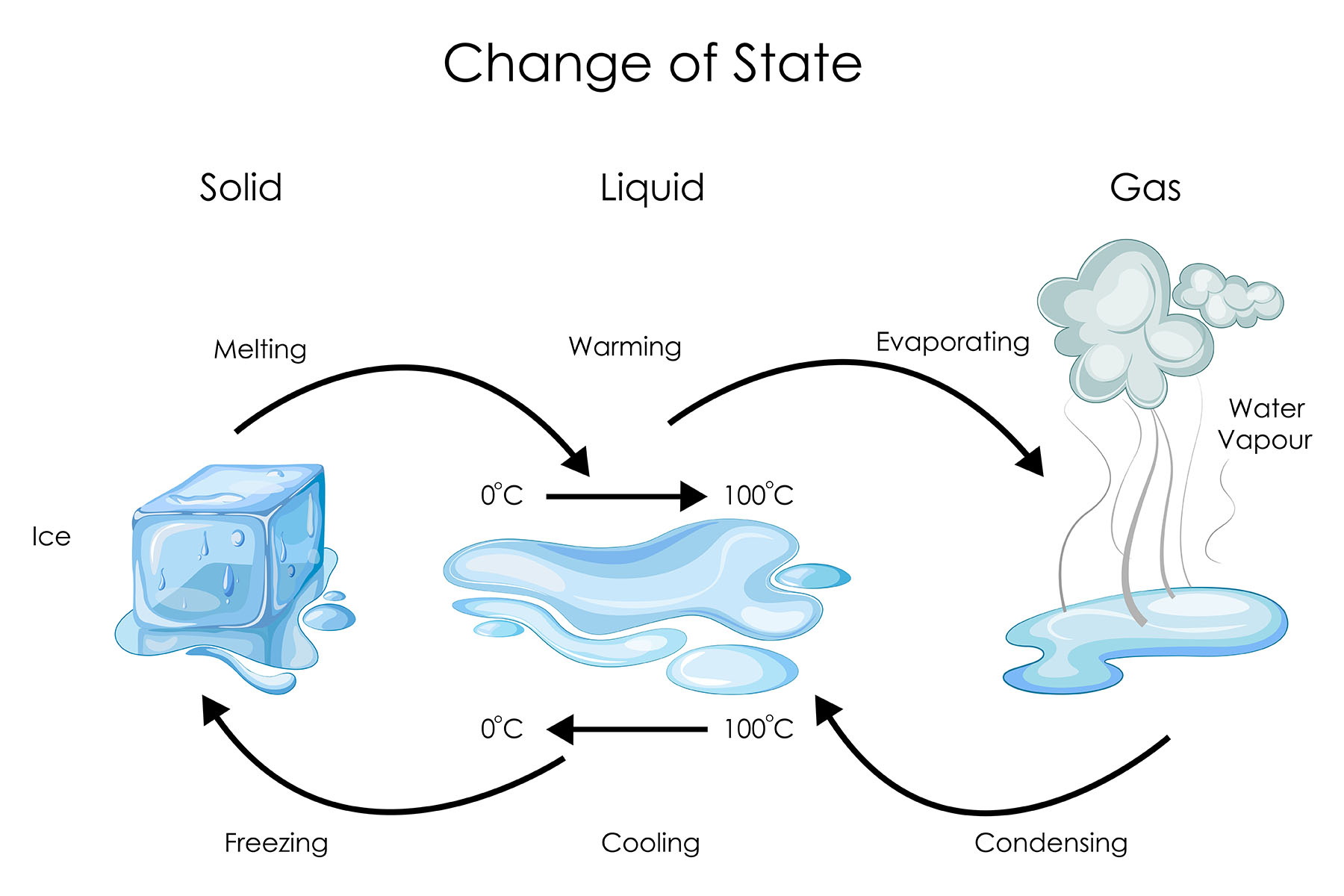
Vapour is a gaseous state of a substance that is below its boiling point. It is formed when the molecules of a liquid or solid have enough energy to overcome the forces of attraction between them.
The amount of vapour in a given space depends on the temperature and pressure of the gas. The higher the temperature, the more vapour can be present. The lower the pressure, the more vapour can be present.
Vapour can be converted back into a liquid by condensation. This happens when the temperature of the vapour is lowered or the pressure is increased.
The fog was a thick vapour that obscured the view.
Noun:
Verb:
The word "vapour" comes from the Old French word "vapor", which also means "a substance in the gaseous state at a temperature below its boiling point".
The first recorded use of the word "vapour" in English was in the 14th century.
Where might you find vapour?
Question:
Define vaporisation and explain how it differs from evaporation. Provide an example of a natural process where vaporisation plays a crucial role.
Answer:
Vaporisation is the phase transition process in which a liquid substance changes into its gaseous state. It involves the absorption of energy, usually in the form of heat, to break the intermolecular forces holding the liquid molecules together. Vaporisation can occur through two main mechanisms: evaporation and boiling.
Evaporation is a specific type of vaporisation that occurs at the surface of a liquid, where molecules with higher kinetic energy escape into the air. It happens at temperatures below the liquid's boiling point and is influenced by factors like temperature, surface area, and humidity.
Boiling, on the other hand, is a more rapid form of vaporisation that occurs throughout the entire liquid when its internal pressure equals the atmospheric pressure. It happens at a specific temperature known as the boiling point.
A natural process where vaporisation plays a crucial role is the water cycle. As sunlight heats up bodies of water like lakes, rivers, and oceans, water molecules at the surface gain enough energy to overcome intermolecular forces and evaporate into water vapour. This vapour rises into the atmosphere, where it cools and condenses to form clouds. Subsequently, the accumulated water droplets in clouds can re-vaporise through processes like sublimation and evaporation, eventually leading to precipitation in the form of rain or snow, which replenishes Earth's water sources.
Address
Developing Experts Limited
Exchange Street Buildings
35-37 Exchange Street
Norwich
NR2 1DP
UK
Phone
01603 273515
Email
hello@developingexperts.com
Copyright 2025 Developing Experts, All rights reserved.