

In chemistry, a solute is a substance that is dissolved in a solvent. A solution is a homogeneous mixture of two or more substances. The solvent is the liquid that dissolves the solute. The solute is the substance that is dissolved in the solvent.
The solubility of a solute is the amount of solute that can be dissolved in a given amount of solvent at a given temperature. The solubility of a solute depends on the chemical properties of the solute and solvent.
For example, sugar is a solute that is soluble in water. This means that the sugar molecules can interact with the water molecules. The sugar molecules are polar, which means that they have a positive and negative end. The water molecules are also polar, so they can interact with the sugar molecules.
Salt is another solute that is soluble in water. Salt is soluble in water because the salt molecules can dissociate into ions. The sodium ions are positive, and the chloride ions are negative. The water molecules can surround the ions, and this helps to dissolve the salt.
The solubility of a solute can be affected by temperature. In general, the solubility of a solute increases with temperature. This is because the molecules of the solute have more energy at higher temperatures, and they are more likely to interact with the solvent molecules.
The solute in a solution of sugar water is sugar.
Noun:
The word "solute" comes from the Latin word "solutus", which means "loosened" or "dissolved".
The first recorded use of the word "solute" in English was in the 19th century.
The word "solute" is derived from the Latin word "solutus", which is itself derived from the Proto-Indo-European word *swel, which also means "loosened" or "dissolved".
What is a solute?
Question:
Define the term "solute" and provide examples of solutes in everyday situations, explaining how solutes interact with solvents to form solutions.
Answer:
A solute refers to a substance that is dissolved in a solvent to form a homogeneous mixture called a solution. In the context of a solution, the solute is the substance present in a smaller quantity that dissolves into a larger quantity of the solvent.
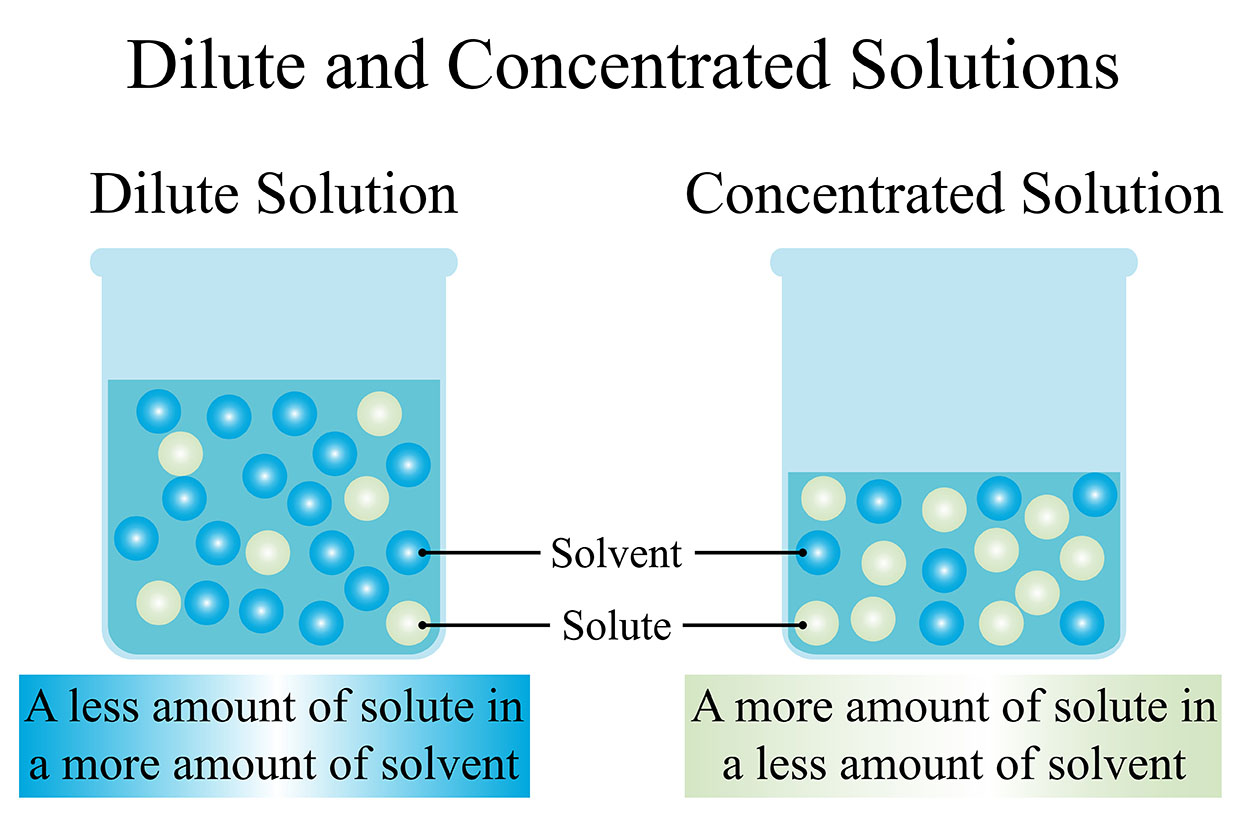
Examples of solutes in everyday situations are abundant. When sugar is stirred into a cup of coffee, sugar acts as the solute, and the coffee itself serves as the solvent. Similarly, when salt is sprinkled into a glass of water, salt acts as the solute while water functions as the solvent. In the preparation of orange juice from concentrated orange juice, the concentrated liquid is the solute, and water serves as the solvent, creating a dilute solution.
The interaction between solutes and solvents is a dynamic process. As solute particles come into contact with the solvent, they are surrounded by solvent molecules, leading to a breaking of intermolecular forces in the solute and a mixing at the molecular level. This process continues until a state of equilibrium is reached, where the rate of solute dissolution is equal to the rate of solute recrystallization.
Address
Developing Experts Limited
Exchange Street Buildings
35-37 Exchange Street
Norwich
NR2 1DP
UK
Phone
01603 273515
Email
hello@developingexperts.com
Copyright 2025 Developing Experts, All rights reserved.