

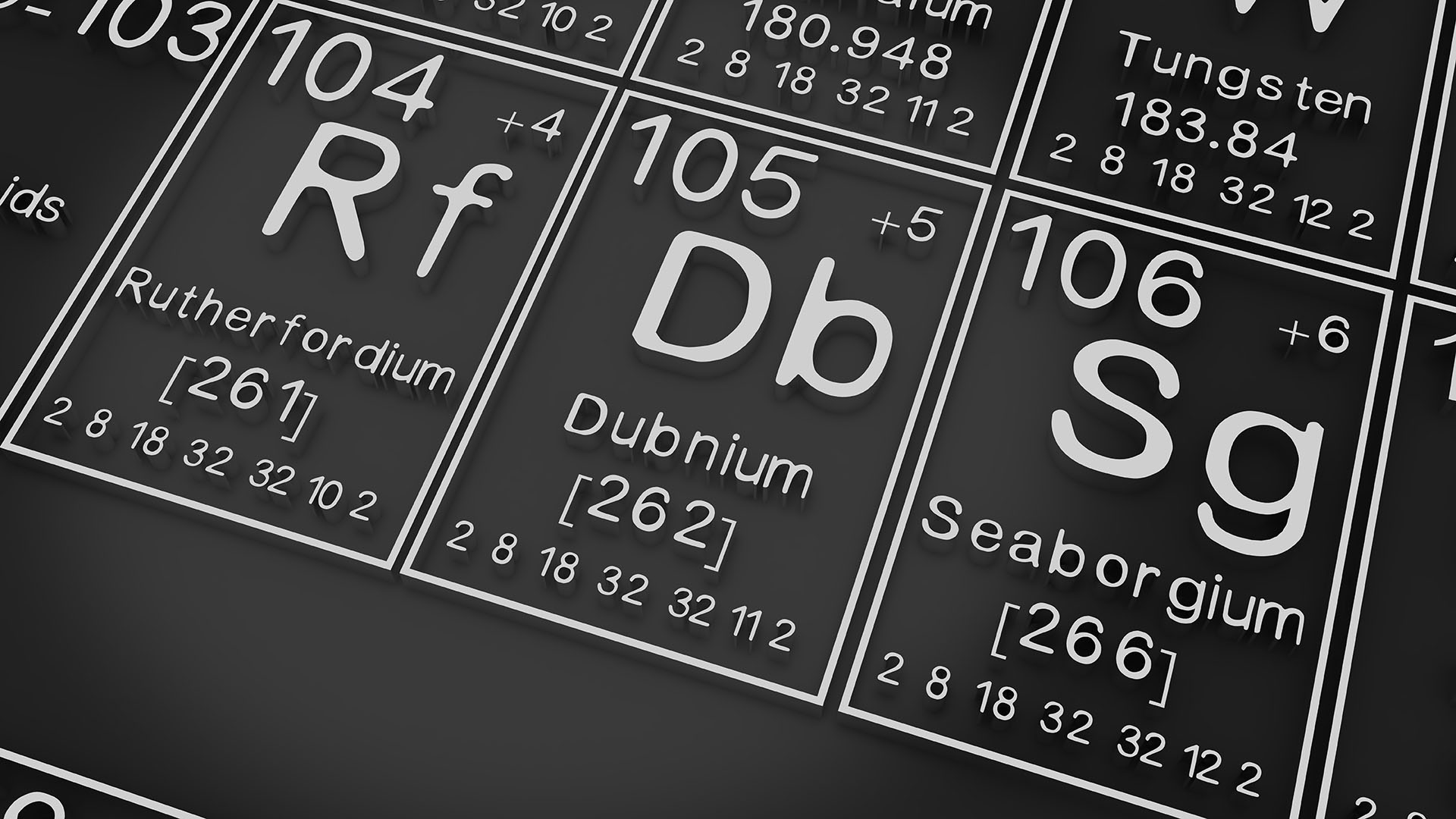
Seaborgium is a chemical element with the symbol Sg and atomic number 106. It is a synthetic element, meaning that it was created in a laboratory and has never been found in nature. Seaborgium is a radioactive metal that is very unstable. It has only been produced in very small amounts and has not yet been studied in much detail.
Seaborgium was discovered in 1974 by a team of scientists at the Lawrence Berkeley National Laboratory in California. The team bombarded americium-243 with neon-22 ions to create seaborgium-253. Seaborgium-253 has a half-life of only 20 seconds, meaning that it decays into other elements in about 20 seconds.
Seaborgium is named after Glenn T. Seaborg, an American nuclear chemist who played a key role in the discovery of many transuranic elements. Seaborgium is the heaviest element that has been named after a living person.
Seaborgium is a member of the actinide series of elements. Actinides are radioactive elements that are found in the periodic table below uranium. Seaborgium is the sixth-heaviest actinide.
Seaborgium is a very rare element. It is estimated that there are only about 100 atoms of seaborgium in the entire universe. Seaborgium is so rare because it is so unstable. It decays into other elements very quickly.
Scientists are still learning about seaborgium. They are trying to understand its properties and its chemical behaviour. They are also trying to find ways to produce more seaborgium so that they can study it in more detail.
Seaborgium is named after Glenn T. Seaborg, an American nuclear chemist.
Noun:
The word "seaborgium" is named after Glenn T. Seaborg, an American nuclear chemist who was instrumental in the discovery of several transuranium elements.
The first recorded use of the word "seaborgium" in English was in 1994.
The word "seaborgium" is an English word, and it is not related to any other languages.
Who was seaborgium named after?
Question:
Explain the significance of seaborgium as a synthetic element and its contributions to our understanding of the periodic table.
Answer:
Seaborgium, a synthetic element with the atomic number 106, holds significance in the realm of nuclear physics and our understanding of the periodic table. It was first synthesized in 1974 through a complex process involving nuclear reactions.
Seaborgium's creation and subsequent study contribute to our knowledge of the transactinide elements, which are elements with atomic numbers higher than uranium (92). By synthesising seaborgium and observing its properties, scientists gain insights into the behaviour of heavy elements, their stability, and their potential applications.
Seaborgium's existence and characteristics are a testament to our ability to create and study elements beyond those found in nature. This expands our understanding of the atomic structure and the trends that govern the periodic table. Additionally, seaborgium is named after the renowned American chemist Glenn T. Seaborg, who made significant contributions to our understanding of the actinide and transactinide series of elements.
While seaborgium's practical applications are limited due to its short half-life, its creation and study have enriched our understanding of nuclear physics and the fundamental properties of matter, contributing to the advancement of science and our exploration of the elements that compose the universe.
Address
Developing Experts Limited
Exchange Street Buildings
35-37 Exchange Street
Norwich
NR2 1DP
UK
Phone
01603 273515
Email
hello@developingexperts.com
Copyright 2025 Developing Experts, All rights reserved.