
pH
Definition
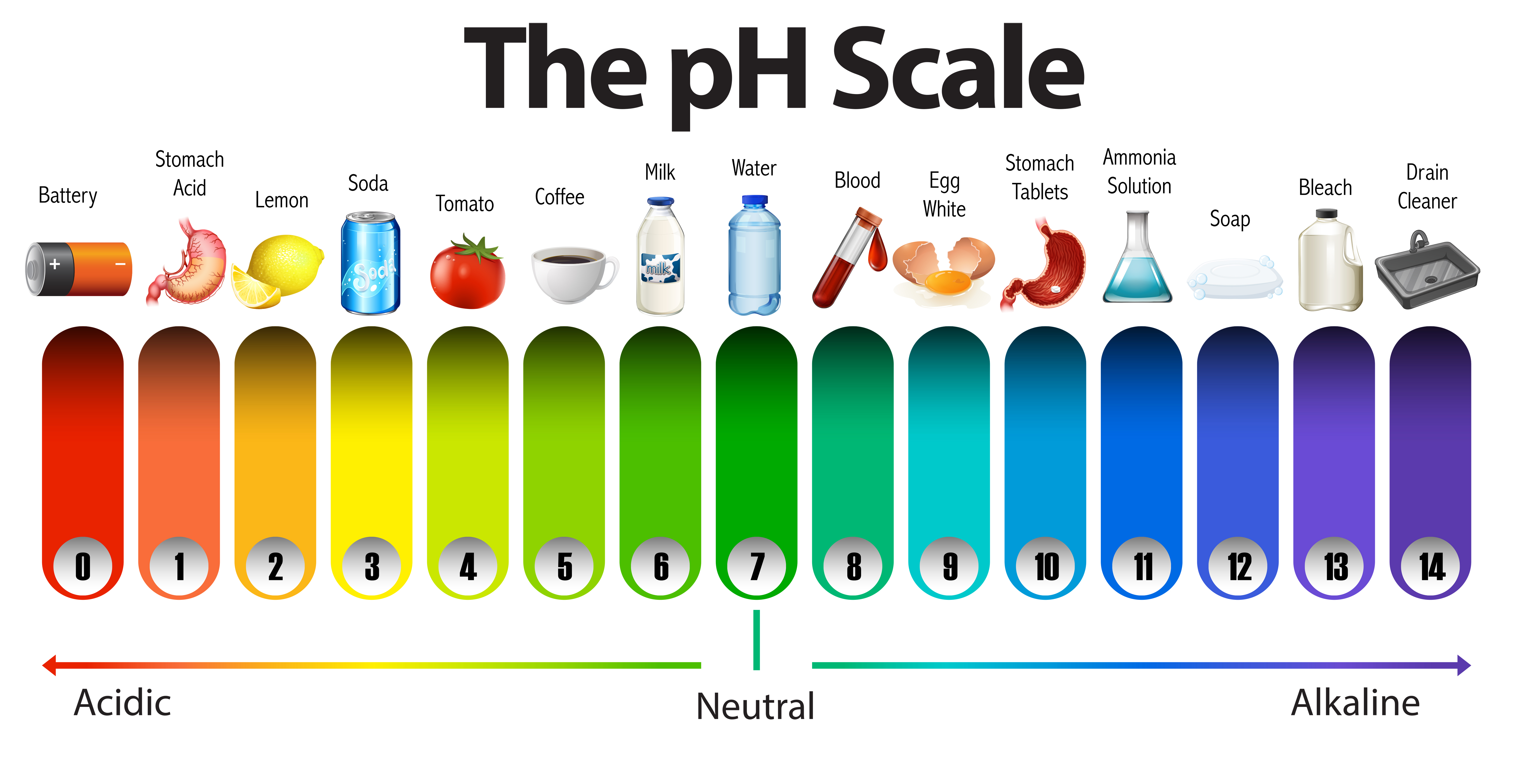
pH is a measure of the acidity or basicity of a solution. It is a logarithmic scale, meaning that each whole number on the scale represents a tenfold difference in acidity or basicity. A pH of 7 is neutral, while a pH less than 7 is acidic and a pH greater than 7 is basic.
The pH of a solution is determined by the concentration of hydrogen ions (H+) in the solution. The more hydrogen ions in a solution, the lower the pH. The fewer hydrogen ions in a solution, the higher the pH.
pH is important in many different areas of science and technology. It is used to measure the acidity of soil, the acidity of food, and the acidity of water. pH is also used to control the reactions in chemical processes and to manufacture products such as soap, detergent, and pharmaceuticals.
How can the word be used?
The pH of a solution can be measured with a pH meter.
Different forms of the word
Noun:
- pH (a measure of the acidity or alkalinity of a solution).
Adjective:
- pH (of or relating to the pH of a solution).
Etymology
The word "pH" is an abbreviation of the German word "Potenz von Hydrogen" (power of hydrogen). The word "Potenz" means "power" and the word "Hydrogen" refers to the hydrogen ion, which is the ion that determines the acidity or alkalinity of a solution.
The word "pH" was first used in 1909 by the Danish chemist Søren Peter Lauritz Sørensen. Sørensen developed the pH scale to measure the acidity or alkalinity of a solution. The pH scale is a logarithmic scale, which means that each unit change on the scale represents a tenfold change in acidity or alkalinity.
Question
What does the pH measure?
AQA Science Exam Question and Answer
Question:
Explain the concept of pH and its significance in understanding the acidity or alkalinity of substances. Provide examples of everyday applications where knowledge of pH is essential and discuss the potential impacts of pH imbalances on living organisms.
Answer:
pH is a measure of the acidity or alkalinity of a solution, indicating the concentration of hydrogen ions present. The pH scale ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are considered acidic, while those above 7 are alkaline (basic).
Understanding pH is crucial in various applications. For instance, in agriculture, soil pH affects plant growth and nutrient availability. In swimming pools, maintaining the proper pH level ensures water safety and prevents irritation. In cooking, the pH of ingredients like vinegar or baking soda can impact the taste and texture of foods.
In biological systems, pH plays a vital role. Human blood, for example, must maintain a pH close to 7.4 for enzymes and cellular processes to function properly. Acidic or alkaline imbalances in the body can disrupt normal physiological functions and lead to health issues.
In aquatic ecosystems, pH levels influence the survival of aquatic organisms. Drastic pH changes can harm fish and aquatic plants, disrupting the delicate balance of the ecosystem.