

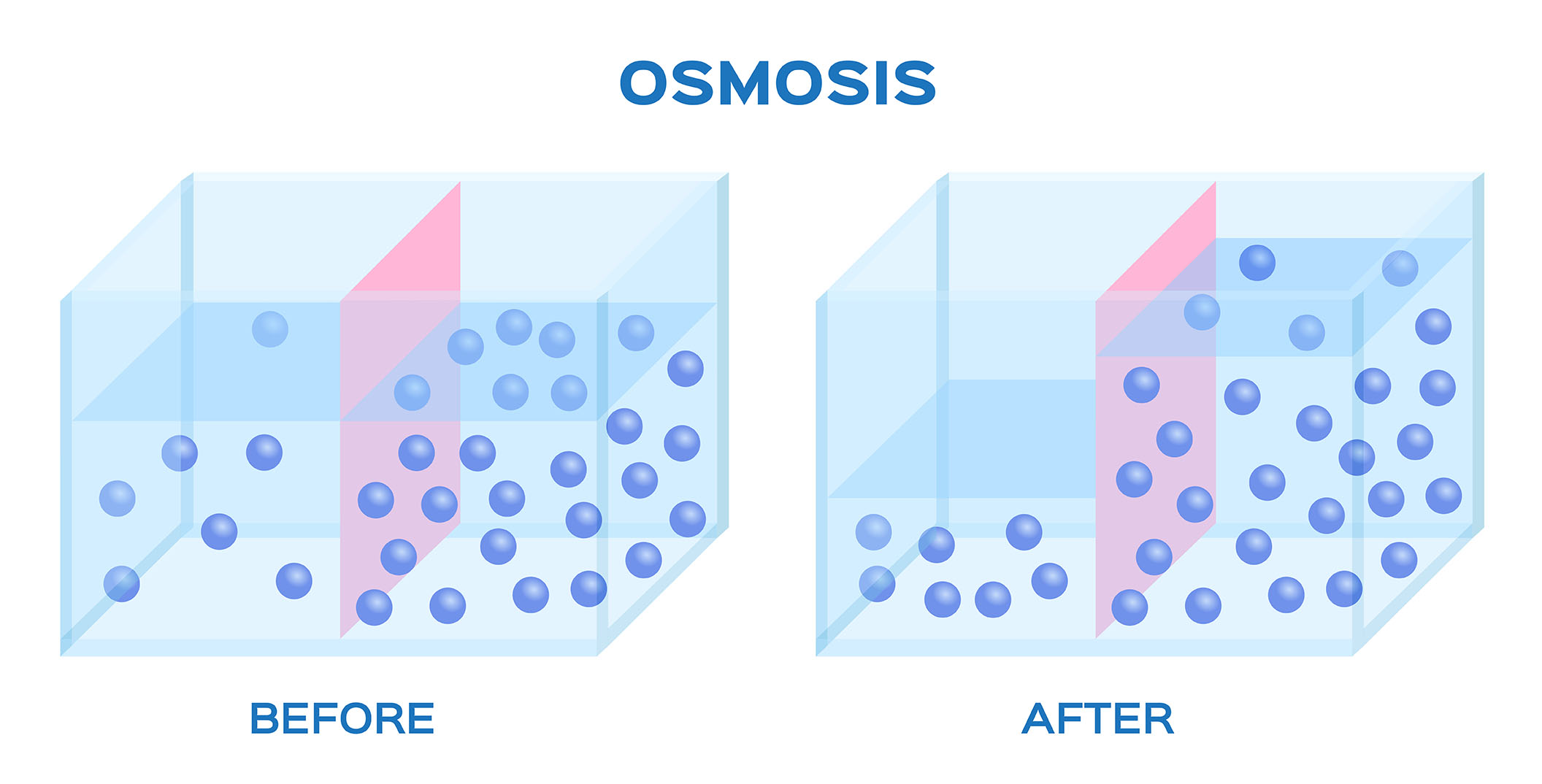
Osmosis is the diffusion of water molecules across a semi-permeable membrane from an area of high water concentration to an area of low water concentration. This process is driven by the difference in water potential between the two areas.
Water potential is a measure of the free energy of water in a system. It is influenced by the solute concentration, temperature, and pressure of the system.
In osmosis, the water potential of the solution with the higher water concentration is lower than the water potential of the solution with the lower water concentration. This creates a driving force for water molecules to move from the solution with the higher water concentration to the solution with the lower water concentration.
Osmosis is a very important process in biology. It is essential for the transport of water and nutrients into and out of cells. It also plays a role in the movement of water in plants and animals.
Here is an example of osmosis in biology:
Osmosis is important for many biological processes, such as the movement of water into and out of cells.
Noun: The diffusion of a solvent through a semipermeable membrane from an area of high concentration to an area of low concentration.
Adjective: Relating to osmosis.
The word "osmosis" comes from the Greek words "en" (in) and "osmos" (thrust).
The word "osmosis" was first used in English in the 19th century. It was used to refer to the diffusion of a solvent through a semipermeable membrane.
What does osmosis mean?
Question:
Explain the process of osmosis in cells and how it relates to the movement of water across cell membranes. Provide a specific example of osmosis in a biological context.
Answer:
Osmosis is the movement of water molecules across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This process is crucial for maintaining the balance of water and solutes within cells and their surrounding environments. Osmosis occurs due to the natural tendency of water to move from areas of high water concentration (low solute concentration) to areas of lower water concentration (high solute concentration) to equalise solute concentrations on both sides of the membrane.
For instance, consider plant cells in a hypertonic solution where the external environment has a higher solute concentration than the cell's cytoplasm. In this case, water will move out of the plant cell through osmosis, causing the cell to lose water and shrink. This can lead to wilting in plants as the lack of water pressure within the cell makes it lose its rigid structure.
In biological systems, osmosis is essential for maintaining cell turgor, allowing cells to function optimally. It also impacts processes like nutrient absorption in the intestines and water regulation in animal cells. Overall, osmosis is a vital mechanism for ensuring proper cellular function and maintaining the delicate balance of solutes and water within organisms.