

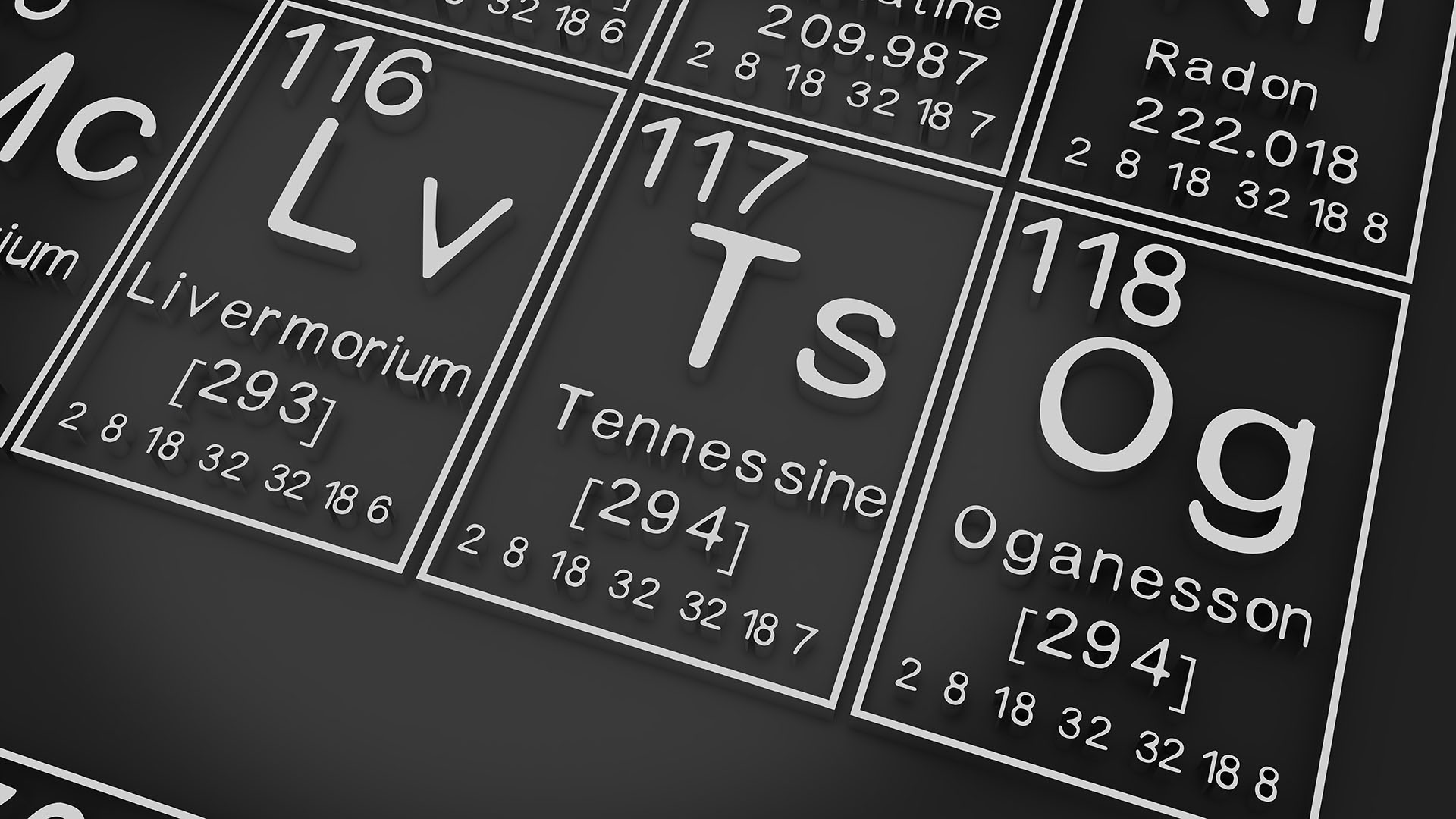
Oganesson is a synthetic chemical element with the atomic number 118. It is the heaviest known element and the first element in the eighth period of the periodic table. Oganesson was first synthesized in 2002 by a team of Russian scientists at the Joint Institute for Nuclear Research in Dubna, Russia.
Oganesson is a highly unstable element with a very short half-life. The longest-lived isotope of oganesson, 294 Og, has a half-life of about 0.6 seconds. Oganesson is thought to be a noble gas, like helium and neon. This means that it is chemically inert and does not react with other elements.
Oganesson is named after Yuri Oganessian, a Russian nuclear physicist who helped to discover it. Oganessian was born in 1933 in Baku, Azerbaijan. He studied physics at the Moscow Engineering Physics Institute and the Kurchatov Institute of Atomic Energy. Oganessian is a leading expert in nuclear physics and has made significant contributions to the study of superheavy elements.
The discovery of oganesson was a major breakthrough in the field of chemistry. It is the first element to be discovered since the 1960s and the first element to be added to the periodic table since 1999. The discovery of oganesson also provides new insights into the structure of the periodic table and the behaviour of superheavy elements.
The name "oganesson" is pronounced as "oh-gah-NES-suhn.".
The word "oganesson" has no different forms. It is a noun that refers to the chemical element with atomic number 118.
The word "oganesson" is named after Yuri Oganessian, a nuclear physicist who led the team that first created the element. Oganessian is a native of Armenia and is considered to be one of the world's leading experts on superheavy elements.
The word "oganesson" was officially approved by the International Union of Pure and Applied Chemistry (IUPAC) in 2016. The IUPAC chose the name "oganesson" in honor of Yuri Oganessian's contributions to the field of nuclear physics.
Who is oganesson named after?
Question:
Explain the significance of oganesson in the periodic table and its role as a superheavy element.
Answer:
Oganesson, with the chemical symbol Og and atomic number 118, is a synthetic element that holds a significant place in the periodic table. It is classified as a superheavy element due to its high atomic number and unstable nature.
Oganesson is part of the noble gas group, located in the far-right column of the periodic table. Its placement showcases its shared properties with noble gases, such as helium and neon. However, oganesson is not naturally occurring; it is created in particle accelerators by colliding with lighter nuclei to form heavier ones.
The significance of oganesson lies in its contribution to our understanding of nuclear physics and the behaviour of extremely heavy elements. Oganesson's short-lived existence—mere milliseconds—limits the possibilities for direct study, but it offers insights into the stability of superheavy nuclei and the potential existence of an "island of stability," a region theorised to contain longer-lived superheavy elements.
Although oganesson doesn't have practical applications due to its extreme instability, its creation and study help expand our knowledge of the fundamental properties of matter and the limits of the periodic table. The research conducted on oganesson and similar superheavy elements enhances our comprehension of nuclear forces and the structure of atomic nuclei.
Address
Developing Experts Limited
Exchange Street Buildings
35-37 Exchange Street
Norwich
NR2 1DP
UK
Phone
01603 273515
Email
hello@developingexperts.com
Copyright 2025 Developing Experts, All rights reserved.