

A molar is a unit of measurement that is used to measure the amount of a substance in a solution. It is equal to the number of moles of a substance in one litre of solution.
A mole is a unit of measurement that is used to count the number of atoms or molecules in a substance. It is equal to the Avogadro number, which is 6.022 x 10^23.
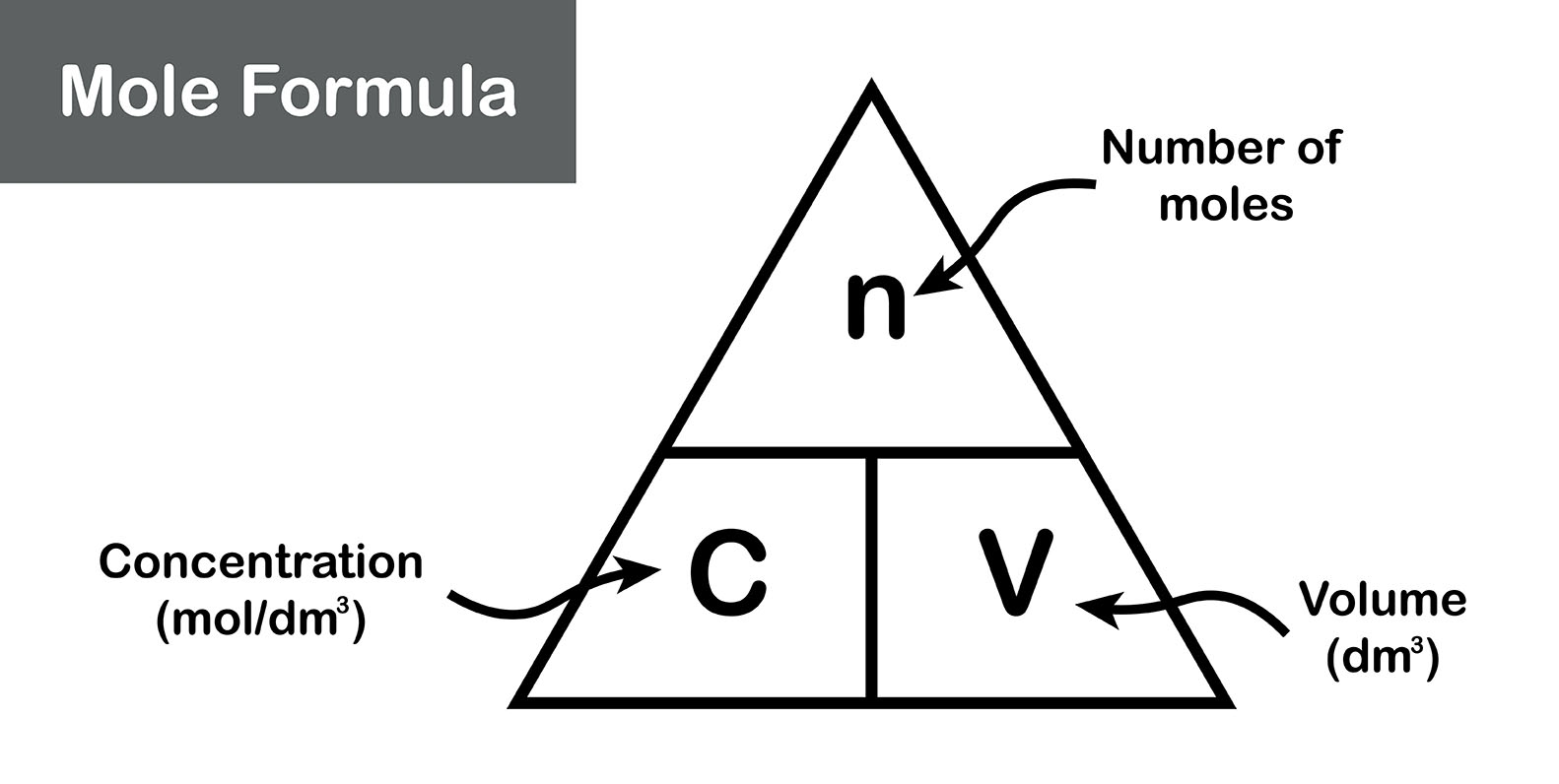
The molarity of a solution is a measure of the concentration of a substance in a solution. It is calculated by dividing the number of moles of the substance by the volume of the solution in litres.
For example, if a solution contains 0.5 moles of sugar in 1 litre of solution, then the molarity of the solution is 0.5 M. This means that there are 0.5 moles of sugar per litre of solution.
Molar concentration is a useful unit of measurement for many different calculations in chemistry. For example, it can be used to calculate the amount of a substance that will react with another substance or the amount of a substance that will dissolve in a given volume of solvent.
The lion used its molars to crush the bones of its prey.
Noun: A tooth with a large, flat surface for grinding food.
Adjective: Relating to or having to do with molars.
The word "molar" comes from the Latin word "mola," which means "millstone." The Latin word "mola" is derived from the Proto-Indo-European root "mel-," which means "to grind.".
The word "molar" was first used in English in the 14th century to describe a tooth with a large, flat surface for grinding food. The word was originally used to compare the teeth to millstones, which are used to grind grain.
What is a Molar?
Question:
Explain the concept of "molar" in chemistry and its significance in quantifying substances. Provide examples of how the molar concept is used to relate mass, moles, and particles in chemical reactions.
Answer:
In chemistry, "molar" refers to a unit of measurement used to quantify the amount of a substance. Specifically, a mole (mol) represents 6.022 × 10^23 particles, which is known as Avogadro's number. This number of particles, whether atoms, molecules, or ions, is the same for all substances.
The concept of the mole is pivotal in connecting mass, moles, and particles in chemical reactions. The molar mass of a substance is the mass of one mole of that substance and is expressed in grams per mole (g/mol). By knowing the molar mass of a compound, scientists can relate the mass of a sample to the number of moles present.
For example, the molar mass of carbon dioxide (CO2) is approximately 44 g/mol. If we have 44 grams of CO2, it corresponds to one mole of CO2 molecules, which is equivalent to 6.022 × 10^23 molecules.
In chemical equations, coefficients indicate the ratio of moles of reactants and products. This allows us to understand the quantitative relationships between substances involved in a reaction.
In conclusion, the concept of "molar" is a fundamental aspect of chemistry, allowing us to relate mass, moles, and particles. It enables scientists to make accurate measurements, predict reaction outcomes, and understand the composition of matter at the atomic and molecular levels.