
joules
Definition
A joule (J) is a unit of energy in the International System of Units (SI). It is defined as the energy transferred to an object when a force of one newton acts on it through a distance of one meter.
In other words, one joule is the work done when a force of one Newton moves an object one meter.
The joule is a relatively small unit of energy. It takes about 4,184 joules to heat one cup of water from room temperature to boiling.
However, the joule is also a very versatile unit of energy. It can be used to measure energy in many different forms, including heat, light, electricity, and mechanical work.
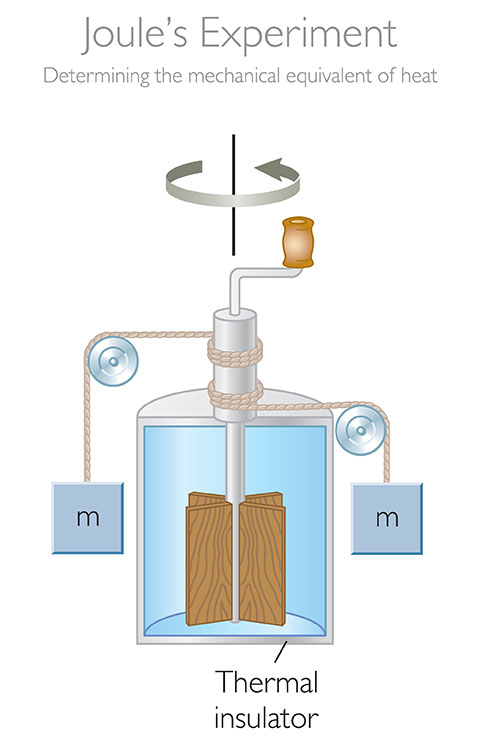
The joule is named after the English physicist James Prescott Joule. Joule conducted a series of experiments in the early 1800s to measure the mechanical equivalent of heat. He found that a certain amount of work always produced the same amount of heat, no matter how it was done.
Joule's work helped to establish the law of conservation of energy, which states that energy can neither be created nor destroyed. It can only be converted from one form to another.
The joule is a fundamental unit of energy in the SI system. It is used in many different fields, including physics, chemistry, engineering, and biology.
How can the word be used?
The joule is a relatively small unit of energy.
Different forms of the word
The word "joule" only has one form. It is the standard unit of energy in the International System of Units (SI). It is named after the English physicist James Prescott Joule.
Etymology
The word "joule" is named after the English physicist James Prescott Joule (1818–1889), who first defined it. Joule conducted a series of experiments in the 1840s to measure the mechanical equivalent of heat. He found that the amount of work required to raise the temperature of a given mass of water by a given amount is equal to the amount of heat that is generated by that work.
The word "joule" comes from the French word "joule", which means "work". It was first used in English in the 1880s.
Question
What is measured in joules?
AQA Science Exam Question and Answer
Question:
What is a joule and how is it used to measure energy? Explain its significance in understanding various forms of energy and their interconversion.
Answer:
A joule is a fundamental unit of measurement used to quantify energy. It represents the amount of energy transferred when a force of one Newton acts on an object to move it one meter in the direction of the force's application. In simpler terms, a joule is a measure of the ability to do work or cause a change.
Joules are widely used to express different forms of energy, including mechanical, thermal, electrical, and more. For instance, the energy stored in a battery, the heat generated by a burner, and the movement of a car are all quantified in joules.
Understanding joules is essential as they provide a common measurement that allows us to compare and analyse various energy sources and their efficiency. They play a crucial role in calculating energy transformations, such as converting potential energy to kinetic energy or electrical energy to mechanical energy.
In today's world, where energy consumption and conservation are paramount, joules enable us to make informed decisions about energy usage. They contribute to our comprehension of sustainability, efficiency, and the potential of different energy sources to power our lives while minimizing environmental impact.