

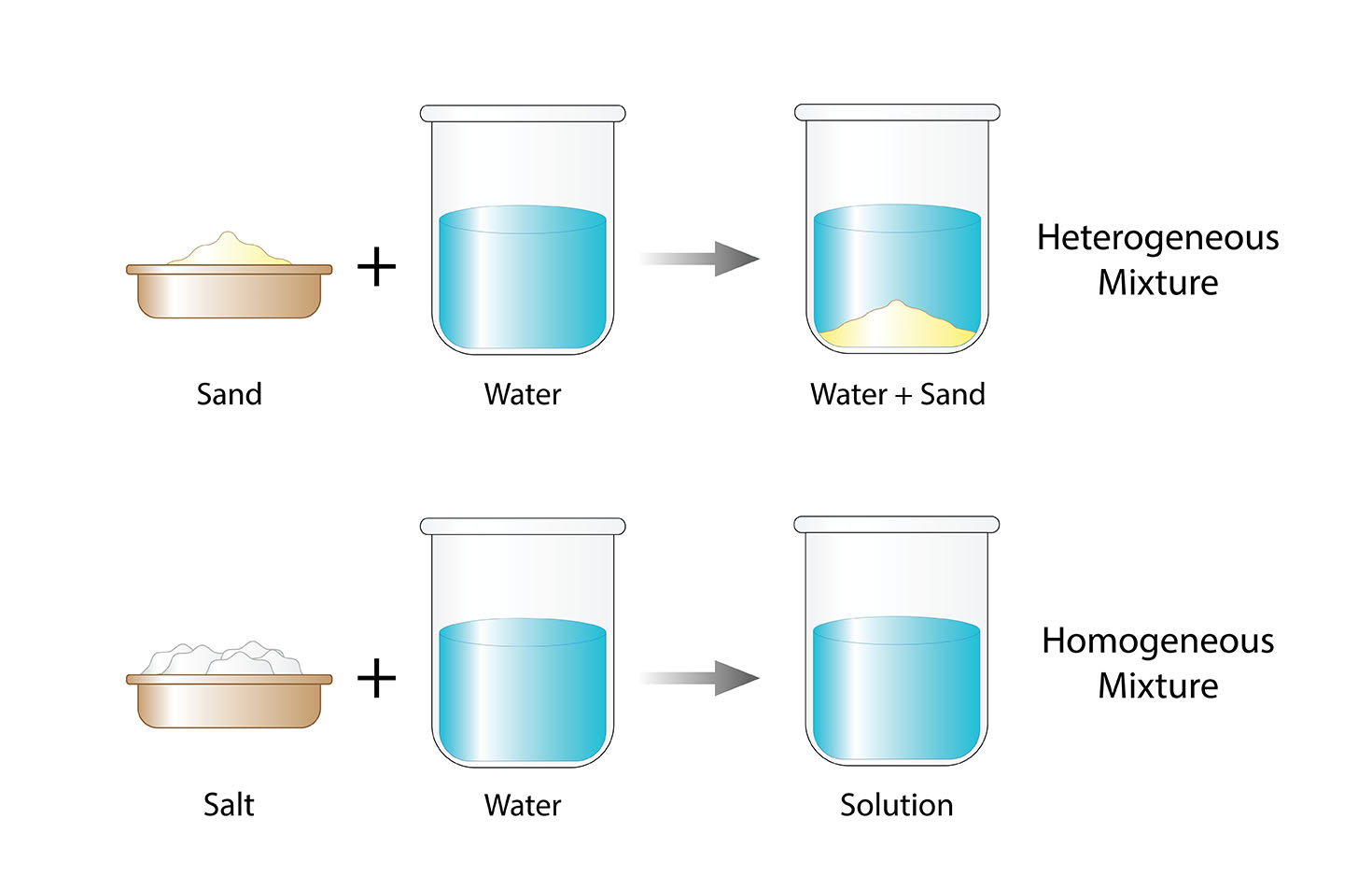
Homogeneous means that something is all the same throughout. A homogeneous mixture is a mixture where the components are evenly distributed throughout the mixture. This means that the composition of the mixture is the same no matter where you sample it.
Homogeneous mixtures are often formed by dissolving a substance in a solvent. For example, when you dissolve salt in water, the salt molecules are evenly distributed throughout the water. This creates a homogeneous mixture.
Other examples of homogeneous mixtures include:
Homogeneous mixtures are often clear or transparent. This is because the components are evenly distributed and there are no large particles that can scatter light.
Homogeneous mixtures are different from heterogeneous mixtures. Heterogeneous mixtures are mixtures where the components are not evenly distributed. For example, a bowl of cereal is a heterogeneous mixture because the cereal pieces are not evenly distributed throughout the bowl.
The mixture was homogeneous.
Noun: homogeneity.
Adjective: homogeneous.
Adverb: homogeneously.
The word "homogeneous" comes from the Greek words "homos", meaning "same", and "genos", meaning "kind". It was first used in English in the 17th century to describe something that is of the same kind or nature.
What does homogeneous mean?
Question:
Explain the concept of a homogeneous mixture and provide examples of everyday substances that demonstrate this property, highlighting the uniform distribution of particles and its significance in various scientific and practical contexts.
Answer:
A homogeneous mixture is a type of mixture in which the components are uniformly distributed throughout, resulting in a consistent composition and appearance. In a homogeneous mixture, it is challenging to visually distinguish the individual substances, as they are thoroughly intermixed on a molecular level.
A classic example of a homogeneous mixture is a solution, such as salt dissolved in water. The salt particles are evenly dispersed throughout the water, creating a uniform distribution. Other everyday substances like air, sugar dissolved in coffee, and vinegar mixed with oil are also examples of homogeneous mixtures.
The uniform nature of homogeneous mixtures is essential in various scientific and practical scenarios. In chemistry, it ensures accurate and consistent reactions, as reactants are uniformly distributed, leading to reliable experimental results. In biology, it allows for the even distribution of nutrients and gases within living organisms. Moreover, in industry and technology, the production of consistent products often relies on homogeneous mixtures.
Understanding homogeneous mixtures and their properties is fundamental to comprehending a wide range of natural and man-made processes. The concept serves as a cornerstone in fields such as chemistry, physics, and materials science, showcasing the significance of uniformity and balance in various contexts.
Address
Developing Experts Limited
Exchange Street Buildings
35-37 Exchange Street
Norwich
NR2 1DP
UK
Phone
01603 273515
Email
hello@developingexperts.com
Copyright 2025 Developing Experts, All rights reserved.