

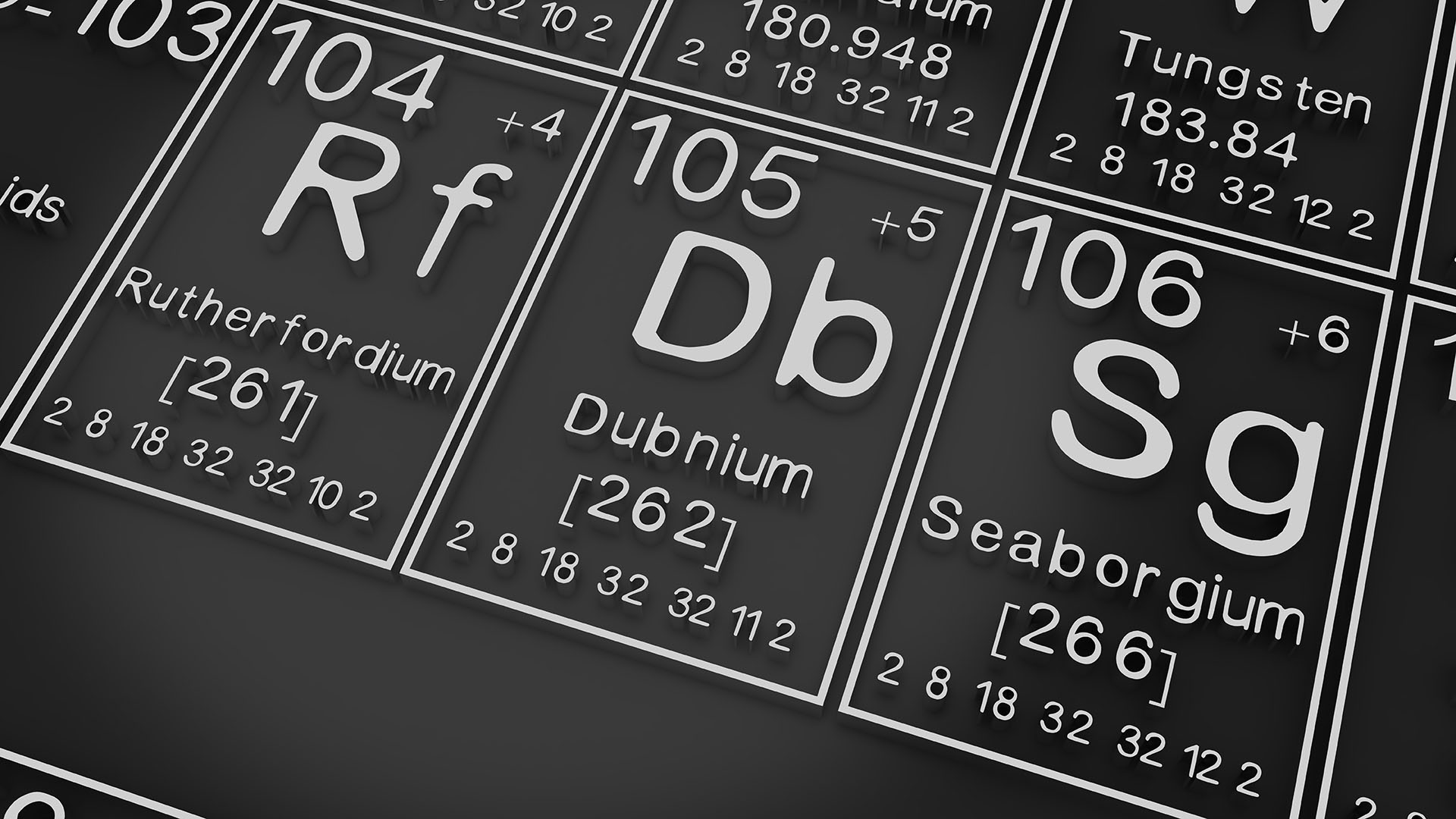
Dubnium is a chemical element with the symbol Db and atomic number 105. It is a synthetic element, meaning that it has never been found in nature. Dubnium was first created in 1968 by a team of scientists at the Joint Institute for Nuclear Research in Dubna, Russia.
Dubnium is a member of the transactinide elements, which are elements with atomic numbers greater than 92. Transactinide elements are very unstable, and they only exist for a very short time. Dubnium has a half-life of about 28 hours, which means that half of the dubnium atoms in a sample will decay into other elements in 28 hours.
Dubnium is a silvery-white metal, and it is thought to be a very reactive element. It is likely to form compounds with other elements, such as oxygen, hydrogen, and chlorine. Dubnium is not currently used for anything, but it may be used in the future for research purposes.
The element symbol for dubnium is Db.
Noun: dubnium.
Adjective: dubnium.
Verb: to dubnium.
Adverb: dubniumly.
The word "dubnium" comes from the Russian city of Dubna, where the element was first synthesized in 1968. The name was officially adopted by the International Union of Pure and Applied Chemistry (IUPAC) in 1976.
What is dubnium?
Question:
Explain the significance of dubnium in the periodic table and its properties as a synthetic element. Describe how dubnium is produced in laboratories and its limited applications due to its high radioactivity. Discuss the role of dubnium in scientific research and its contribution to our understanding of the periodic table and the behaviour of heavy elements.
Answer:
Address
Developing Experts Limited
Exchange Street Buildings
35-37 Exchange Street
Norwich
NR2 1DP
UK
Phone
01603 273515
Email
hello@developingexperts.com
Copyright 2025 Developing Experts, All rights reserved.