
atom
Definition
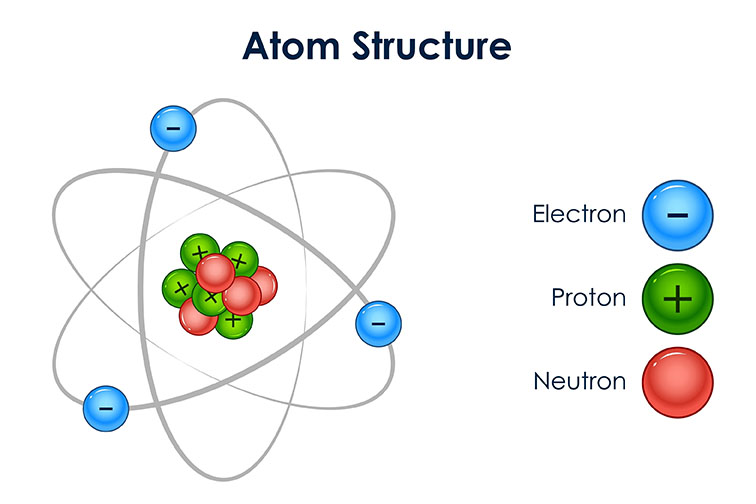
An atom is the basic unit of matter that forms all elements. It is made up of three subatomic particles: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus of the atom, while electrons orbit the nucleus.
The number of protons in an atom's nucleus determines the element that the atom is. For example, all atoms with one proton are hydrogen atoms, all atoms with two protons are helium atoms, and so on.
The number of neutrons in an atom's nucleus can vary, which is why there can be different isotopes of the same element. For example, there are three isotopes of hydrogen: protium, deuterium, and tritium. Protium has no neutrons, deuterium has one neutron, and tritium has two neutrons.
Electrons orbit the nucleus of the atom in shells. Each shell can hold a certain number of electrons, and the electrons in the outermost shell determine the chemical properties of the atom.
Atoms can combine to form molecules. A molecule is a group of two or more atoms that are bonded together. The type of bonding that occurs between atoms determines the properties of the molecule.
How can the word be used?
Atoms are made up of protons, neutrons, and electrons.
Different forms of the word
Noun: atom.
Adjective: atomic.
Adverb: atomistically.
Synonyms: molecule, particle, ion, element.
Antonyms: compound.
Etymology
The word "atom" comes from the Greek word átomos, which means "indivisible." The Greek word is made up of the prefix a, which means "not," and the noun tomos, which means "cut.".
Question
What can vary in an atom's nucleus?
AQA Science Exam Question and Answer
Question:
What is the structure of an atom and what are the different parts of an atom?
Answer:
An atom is the basic unit of matter that forms all elements. It is made up of three subatomic particles: protons, neutrons, and electrons. Protons and neutrons are found in the nucleus of the atom, while electrons orbit the nucleus.